Abstract
We used a behavioral test to investigate long-term consequences of neonatal noxious stimulus in the anxiety-like behavior and describe differences between males and females. Male and female Wistar rats were submitted to either tactile (control groups) or noxious stimulus (pain groups) since birth, for 15 days and were followed up to 6 months of life. Experiments were performed on days 15, 30, 90 and 180 after birth. Rats of different groups, ages and genders were exposed only once to the elevated plus-maze (EPM), an apparatus largely used to detect anxiety-related behaviors in rats. For the open arms of the EPM, control animals showed an increase in the number of entries from 15 to 30 days of age followed by a decrease of this number at older ages. The comparison between treatments (control and pain) showed, for males, a reduced number of entries in the open arms in the pain group at 15 and 30 days and the opposite situation at 180 days. No differences were found between pain and control groups in females. Our results are in agreement with the literature that shows sex-dependent changes following chronic stress; stress being anxiolytic in males and anxiogenic in females. We point to the fact that acute painful stimulus in the neonatal period caused persistent changes in anxiety-like behavior in the adult life, independently of previously described intrinsic gender differences on memory, task performance, attention bias or other behaviors.
Author Contributions
Academic Editor: João Guilherme de Moraes Pontes, absent, Brazil.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Nathalia Leilane Berto Machado, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Chronic pain is described as an important debilitating phenomenon, affecting approximately 20% of the population1. In addition to this statistics, the described prevalence of anxiety disorders among patients with chronic pain may range between 20 to 40%2,3. In the past few years, several studies investigated differences in the way that males and females experience pain4,5,6,7,8,9. In fact, the prevalence of chronic musculoskeletal pain is described to be higher among women10,11. Also, men and women respond differently to pain treatments12,13,14. Laboratory research in controlled environment show that women are usually more sensitive to pain15,16,17,18,19,20, report higher pain intensity and more often report widespread pain21,22,23,24.
Neonatal pain and its chronic effects have been receiving more attention over the last decade particularly due to advances in the perinatal medical care and the increased survival rate of infants born premature4. There is growing clinical evidence that exposure to noxious stimulation in neonatal period leads to long-term alterations in behavioral responses to either tactile or noxious somatosensory stimulation25,26,27. In animal models, neonatal noxious stimulation is associated with long-lasting changes in somatosensory structures and function28,29,30,31,32,33.
Despite the amount of literature on pain sensitivity and long term structural alterations in different areas of the nervous system33,34after noxious stimulus in early life, long-term anxiety-like behavior and differences between males and females need to be further explored. Thus, we used a behavioral test to investigate long-term consequences of neonatal noxious stimulus in the anxiety-like behavior and describe differences between males and females.
Material and Methods
Animals
All procedures performed in this study were approved by the Institutional Ethics Committee for Animal Research (CETEA - Comitê de Ética em Experimentação Animal, protocol number 044/2010) from the School of Medicine of Ribeirão Preto, University of São Paulo, and were carried out according to the guidelines of the International Association for the Study of Pain (IASP) and National Institutes of Health (NIH).
Female Wistar rats were accompanied throughout pregnancy. All animals were maintained on 12 hour light/dark cycle, controlled temperature and relative air humidity and noise, with food and water ad libitum. Immediately after birth, each litter was randomly assigned in pain groups or control groups. Pain groups received noxious stimuli that consisted of rapid insertions of a 30-gauge needle into the plantar foot pad and the lateral surface of the right hind paw (15 insertions), twice per day for 15 days, starting at birth. Control groups received tactile stimuli with a cotton-swab into the plantar and the lateral surface of the right paw twice per day for 15 days starting at birth. This protocol was adapted from Anand et al.35 and used in previous studies from our laboratory33,34,36.
A total of 157 neonate Wistar rats (Rattus norvegicus) were divided into 16 groups, being 8 control groups and 8 pain groups. Control or pain animals were studied at 15, 30, 90 and 180 days of life and subdivided by gender, as shown on Table 1.
Table 1. Number of animals studied according to group, gender and age.| Male | Female | |||
| Age | Control | Pain | Control | Pain |
| 15 days | 10 | 10 | 10 | 10 |
| 30 days | 10 | 10 | 10 | 10 |
| 90 days | 9 | 10 | 9 | 9 |
| 180 days | 10 | 10 | 10 | 10 |
Behavioral Test
The behavioral test was performed using the Elevated Plus-Maze (EPM). This apparatus consist of two open arms (50 x 10 cm) and two enclosed arms (50 x 10 x 40 cm) with an open roof, connected by a central platform (10 x 10 cm) and elevated 50 cm from the floor7,37.
Before evaluation, the animals were weighed and then remained in the experiment room in their cages for at least 15 minutes so they could get adapted to the environment. Afterwards, they were placed for 5 minutes in the EPM and recorded with a video camera. Each animal was exposed to the EPM only once so they would not get used to the apparatus.
At the beginning of the test the animals were placed individually on the central platform of the maze, with the head turned to one of the open arms. Each entry in one of the arms, the time spent in the open or the closed arm as well the time spent on the central platform (time to make a decision) was recorded, counted and analyzed by the software X-Plot Rat. An entry in one of the arms was computed when the animal placed all four feet within the specific arm.
Data Analysis and Statistics
The Sigma Stat software, version 3.01 (Jandel Scientific) was used for the analysis. The values were expressed as mean ± SEM.
Statistical analysis was performed by unpaired t-test for comparisons between two groups (males x females, same age and control x pain groups, same age). Different ages were compared by the one-way ANOVA, followed by Holm-Sidak post-test. The interaction between ages and treatments was investigated by the repeated measure two-way ANOVA, followed by Duncan’s test.
Statistical significance was considered if p values were equal or smaller than 0.05.
Results
Body Weight
Body weight data from all groups is shown in Table 2. Body weight increased significantly with age in all groups studied independently of gender or treatment (male control and pain, and female control and pain). Also, males were heavier than females at 90 and 180 days of age, in control and pain groups.
Table 2. Body weight according to group, gender and age.| Male | Female | |||
| Age | Control | Pain | Control | Pain |
| 15 days | 33 ± 1 | 31 ± 2 | 34 ± 2 | 36 ± 1+ |
| 30 days | 135 ± 15 | 117 ± 5 | 116 ± 13 | 114 ± 6 |
| 90 days | 453 ± 16* | 446 ± 18* | 337 ± 15*+ | 305 ± 9*º+ |
| 180 days | 622 ± 19*# | 552 ± 25*#º | 384 ± 18*#+ | 357 ± 10*#+ |
The comparison between pain and control groups showed that pain group animals presented smaller body weight at ages of 90 days for females (p = 0.028) and 180 days for males (p = 0.001), with a non-significant (p = 0.068) smaller weight in females with this age.
Number of Entries in the Open Arms
The number of entries in the open arms of the EPM for all experimental groups is shown in Figure 1, upper panels.
Figure 1.Number of entries and percentage of the total time spent in the open arms of the elevated plus maze. Data expressed as mean ± SEM. * indicates significant difference compared to 15 days of age, same group. # indicates significant difference compared to 30 days of age, same group. º indicates significant difference compared to control group, same age and gender. + indicates significant difference between gender, same age and treatment.
The comparison between ages on control animals showed an increase in the number of entries from 15 to 30 days of age followed by a decrease of this number at older ages. For males, significant differences were detected between 15 and 30 days (P = 0.009) and between 30 and 180 days (p = 0.009). For females, differences were detected between 15 and 30, 30 and 90 and 30 and 180 days (p = 0.001). For the pain groups, males showed a similar pattern described for controls but the increased number of entries was detected at 90 days of age, with difference between 15 and 90, 15 and 180 and 90 and 180 days (p = 0.002). For the females of the pain group, no significant differences were observed with aging (p = 0.160).
The comparison between genders on control groups showed no significant difference in all ages studied. On pain groups, gender differences were detected only at 15 days of age, being the number of entries in the open arm in females larger than males (p = 0.006).
The comparison between treatments (control and pain) showed, for males, a tendency for a reduced number of entries in the open arms in the pain group at 15 (p = 0.078) and 30 (p = 0.092) days and the opposite situation at 180 days (p = 0.05). No differences were found between pain and control groups in females.
Time Spent in the Open Arms
The percentage of the time that the animals spent in the open arms of the EPM from all experimental groups is shown in Figure 1, lower panels. As expected, results are in line with those for the number of entries in the open arms. The most important difference was that males from pain group at 180 days of age spent more time in the open arms of the EPM compared to all other ages. They also spent more time in the open arms compared to controls of the same age. For the females, 30 days old animals spent more time in the open arms of the EPM that animals with 15, 90 and 180 days on the control group while no differences were observed for the pain group.
Number of Entries in the Closed Arms
The number of entries in the closed arms of the EPM from all experimental groups is shown in Figure 2, upper panels.
Figure 2.Number of entries and percentage of the total time spent in the closed arms of the elevated plus maze. Data expressed as mean ± SEM. * indicates significant difference compared to 15 days of age, same group. # indicates significant difference compared to 30 days of age, same group. & indicates significant difference compared to 90 days of age, same group. º indicates significant difference compared to control group, same age and gender. + indicates significant difference between gender, same age and treatment.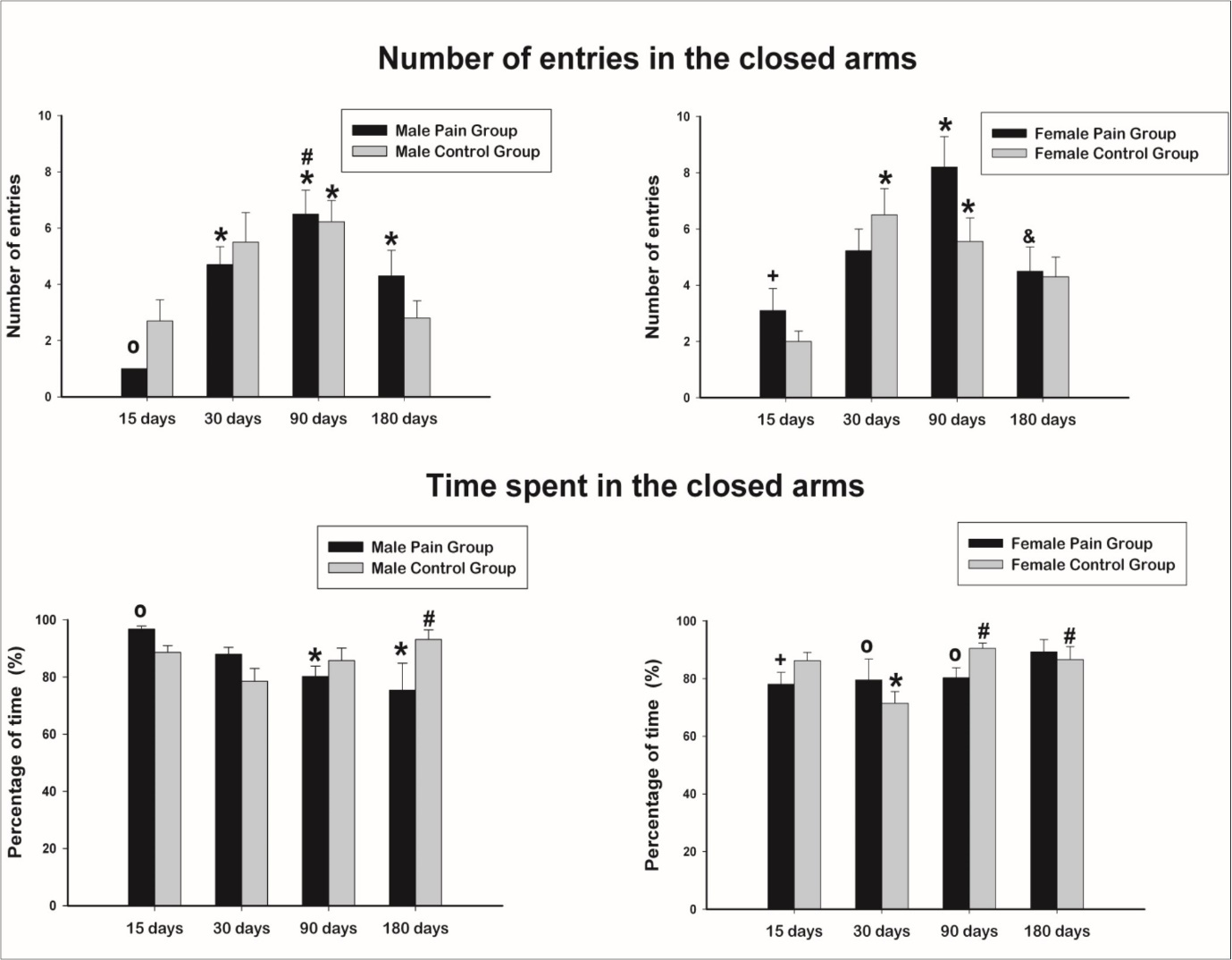
The comparison between ages on control animals showed an increase in the number of entries from 15 to 30 days of age followed by a decrease of this number in older ages. For males, significant difference was detected between 15 and 30 days. For females, differences appeared between 15 and 30 and 30 and 90 days. For the pain groups, males showed a similar pattern described for controls but the increased number of entries was detected at 90 days of age, with differences between 15 and 30, 15 and 90 and 15 and 180 days. For the females in the pain group, a difference was present only between 15 and 90 days, also with the 90 days animals showing the larger values compared to the other ages.
The comparison between genders on control groups showed no significant difference in all ages studied. On pain groups, gender differences were detected only at 15 days of age, being the number of entries in the closed arm in females larger than males.
The comparison between treatments (control and pain) showed, for males, a reduced number of entries in the closed arms in the pain group at 15 days (p = 0.015) with no differences for the other ages. No differences were found between pain and control groups in females.
Time Spent in the Closed Arms
The percentage of the time that the animals spent in the closed arms of the EPM from all experimental groups is shown in Figure 2, lower panels. Interestingly, the more time the animals entered in the closed arms, the less time they spent in these arms, in all groups and ages studied. This is the opposite behavior of the animals in the open arms as the larger number of entries in those arms, the larger the time they spent in there.
The comparison between ages on control animals showed an increased time spent in the closed arms at 180 days compared to 30 days of age. For females, animals at 90 days and at 180 days spent more time in the closed arms compared to 30 days old animals. For the pain groups, male animals at 90 days and at 180 days spent less time in the closed arms compared to 15 days old animals while no differences were observed between female pain groups.
The comparison between genders on control groups showed no significant difference in all ages studied. On pain groups, gender differences were detected only at 15 days of age, being the time spent in the closed arm in females smaller than males.
The comparison between treatments (control and pain) showed, for males, an increase in the time spent in the closed arms in the pain group at 15 days (p = 0.005) with no differences for the other ages. For the females, differences were detected on ages 30 and 90 days, with opposite behaviors. While 30 days old controls spent less time in the closed arms compared to the pain animals, 90 days old controls spent more time in the closed arms compared to the pain animals.
Time Spent in the Central Platform
Male and female control animals reduced the time spent in the central platform with aging in a very similar way. The large difference observed was between ages 30 and 90 days, in both genders. No differences between genders were observed between the controls.
Females from the pain groups showed a similar pattern of reduction of the time spent in the central platform (difference found between 15 and 180 days old animals), but with higher values compared to female controls in all ages. Statistical significance between control and pain groups was attained only at 15 days, while for the other ages only a trend towards higher values was observed.
Male pain animals showed an erratic behavior when analyzing the time spend in the central platform. Younger animals (15 days old) spent significantly less time in the central platform compared to controls but increased this time up to 90 days of age, then decreased again at 180 days. No differences between ages were detected.
Discussion
There are neural circuits and connections involved both in pain and anxiety and innate fear. It is known that dorsal periaqueductal gray matter play a role in descendent modulation of pain but it also receive projections from the amygdala, being an important structure on defensive behavior38,39. Thus, we hypothesized that painful stimulus could affect behavioral circuits producing, perhaps, an anxiety-like behavior. The elevated plus-maze (EPM) is an apparatus largely used to detect anxiety-related behaviors in rats and it is described that a lower number of entries and time spent in the open arms suggest an anxiety-like behavior37.
Pain and Anxiety-Like Behavior
An increasing number of studies4,33,34,35,40have been performed to investigate whether painful stimuli during neonatal period can trigger behavioral changes in a short period of time or if these changes even become persistent in the long-term. Our findings suggest that painful stimuli in neonatal period may affect males in a short period of time (15 and 30 days of age), triggering an anxiety-like behavior demonstrated by a lower number of entries and time spent in the open arms of EPM associated with a longer time spent in the closed arms when compared to control group. Also, young males spent less time in the central platform of the EPM, suggesting that they take less time to make the decision to seek for shelter in the closed arms. For the females, right after after the painful stimulus, there was no difference compared to controls but they showed more entries and also spent a longer time in the open arms of the EPM compared to males from the pain group. It was previously shown that male young rats have lower exploratory behavior when exposed to the EPM test, compared to females41. In our study, this expected behavior was not influenced by pain at short term.
On an interesting way, after 180 days post-pain stimulus, male animals showed a change on the behavior, with an increased number of entries and increased time spent in the open arms of EPM, presenting a more active and exploratory behavior and an increased mobility compared to controls. This behavior might be related to a possible attentional bias similar to that described for humans suffering from chronic pain42,43.
This behavior was significantly different from male controls and also significantly different when compared to females from the pain group. Females, after 180 days post-painful stimulus showed a smaller number of entries on the opened arms of the EPM and spent more time in the closed ones, suggesting that, at long term, painful stimuli induced an anxiety-like behavior in females. Female rats exhibit enhanced hyperalgesia in adulthood compared to neonatal injured males44. Also, Negrigo et al.6 showed that females became more anxious than controls and also compared to males, after nociceptive neonatal stimuli. Both results are in agreement to ours, independently of the nociceptive stimulus that were applied in each different experiment.
In short term, male and female that received painful stimuli during neonatal period showed a lower number of entries and time spent in the open arms when compared with the respective control groups. Parent et al.1 reported similar results using an inflammatory pain model in male adults, showing an increased anxiety-like behavior 29 days after pain induction, corroborating the hypothesis that pain experience may be anxiogenic for males in the short term.
We showed that males from pain and control groups showed a lower number of entries and spent less time in the open arms than females from pain and control group, suggesting that males, regardless the treatment, present more pronounced anxious-like behavior than females. Studies in humans45,46,47indicate that women suffer twice more of anxiety and mood disorders than men, being both disorders associated with stress. However, the type of stressor play a role on how pain can be experienced by genders; men are more exposed to physical attacks and severe accidents while women are more vulnerable to sexual abuse and interpersonal assaults, being the latter more related to affective and emotional aspects of pain, while the first are more related to the sensory component of pain. Thus, this is suggestive that painful stimuli as a physical aggression to the sensory system may affect more males that females.
Aging and Anxiety-like Behavior
Aging is associated with increased anxiety-like behavior in rats48. Neoanatal pain exacerbated this behavior in females but not in males. Male rats are known for better acquisition and retention of spatial tasks49. This ability might have influenced our results in older males since there was a significant difference compared to females. We assessed animals of different ages representing different developmental stages, from neonatal to adulthood and intriguing information was obtained from the control groups in both genders.
According to Sengupta50, rats with 15 days of life can be considered neonates. At this age, males performed less entries in the open arms, suggesting less mobility/anxiety compared with females of same age. Frankola et al.51 showed that male rats separated from mothers were impaired in their non-spatial and spatial memory and other tasks, compared to females. The authors discuss the possible limited influence of gonadal hormones since the rats were prior to puberty at this age. In our study, rats were separated from their mothers for either painful or tactile stimulations, creating similar conditions for both genders independently of treatment. Thus, our results do not differ from those obtained by Frankola et al.51, indicating an intrinsic gender difference in behavior at this period of life.
When animals reach 30 days of life they can be compared to children since they were weaned at 21 days but have not reached sexual maturity yet50,52. At 30 days of life, both males and females of control groups performed more entries and spent more time in the open arms compared with older ages. This behavior is explained by a higher motor activity usually present in young rats, associated with the increased exploratory behavior. Intrinsic gender differences were not observed in the behavior of our animals. Nevertheless, females of pain group performed a lower number of entries and spent less time in the open arms compared to control group indicating that painful stimuli decreased the females’ exploratory behavior acutely and this is becoming persistent after the stimulus in no longer being applied.
Animals with 90 days of life can be treated as young adults50,52 and 180 days old animals can be considered adults50. The 90 days old females showed a smaller number of entries and spent less time in the open arms of the EPM compared to animals with 30 days of age. A similar behavior was observed for male animals. But, when animals reached 180 days of life, males behaved differently from females, spending more time in the closed arms of the EPM. This seems to be an intrinsic gender behavior difference.
Anxiety-related behavior measures showed that males are generally more anxious and that stress increases male, but decreases, female anxiety-related behaviors53. In our study, the stress factor (painfull stimulus) was no longer present at adult ages and sex differences were still present in both groups: pain and tactile neonatal stimulus.
Conclusions
Our results are in agreement with the literature that shows sex-dependent changes following chronic stress; stress being anxiolytic in males and anxiogenic in females54. But we point to the fact that acute painful stimulus in the neonatal period caused persistent changes in anxiety-like behavior in the adult life, independently of intrinsic gender differences on memory, task performance, attention bias or other behaviors.
Acknowledgements
The authors thank Mr. Antônio Renato Meirelles e Silva, Experimental Neurology Laboratory, School of Medicine of Ribeirão Preto, for his excellent technical support. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (Grant numbers 2004/01390-8, 2009/50389-6, 2009/16748-9, 2010/12518-6, 2012/00321-0, 2013/20549-7 and 2018/10474-3); Conselho Nacional de Pesquisa e Tecnologia - CNPq (Grant number: 301333/2017-3); and Fundação de Apoio ao Ensino e Pesquisa do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto - FAEPA (Grant number: 393/2005). There is no conflict of interest to declare by all authors.
References
- 1.Parent A J, Beaudet N, Beaudry H, Bergeron J.Bérubé P et al (2012).Increasedanxiety-like behaviors in rats experiencing chronic inflammatory pain.Behav. , Brain Res 229, 160-167.
- 2.Kessler R C, Chiu W T, Demler O, Merikangas K R, Walters E E. (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. , Arch Gen Psychiatry 62, 617-627.
- 3.Twillman R K. (2007) Mental disorders in chronic pain patients. , J Pain Palliat Care Pharmacother 21, 13-19.
- 4.LaPrairie J L.Murphy AZ (2010)Long-term impact of neonatal injury in male and female rats: Sex differences, mechanisms and clinical implications.Front. , Neuroendocrinol 31, 193-202.
- 5.Hashmi J A.Davis KD (2010)Effects of temperature on heat pain adaptation and habituation in men and women.Pain 151:. 737-743.
- 6.Negrigo A, Medeiros M, Guinsburg R.Covolan L (2011)Long-term gender behavioral vulnerability after nociceptive neonatal formalin stimulation in rats.Neurosci. , Lett 490, 196-199.
- 7.Luo J, Wang T, Liang S, Hu X, Li W.(2013)Experimental gastritis leads toanxiety- and depression-like behaviors in female but not malerats.Behav Brain Funct 9:. 46.
- 8.Mansfield K E, Sim J, Jordan J L, Jordan.KP (2016)A systematic review and meta-analysis of the prevalence of chronic widespreadpainin the general population.Pain 157:. 55-64.
- 9.Liu X K, Xiao S Y, Zhou L, Hu M.Liu HM (2018)Different predictors ofpainseverity across age andgenderof a Chinese rural population: a cross-sectional survey.BMJ Open 8:e020938.
- 10.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher.D (2006)Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment.Eur. , J Pain 10, 287-333.
- 11.Stubbs D, Krebs E, Bair M, Damush T, Wu J.et al (2010)Sex differences inpainandpain-related disability among primary care patients with chronic musculoskeletalpain.Pain. , Med 11, 232-239.
- 12.Riley JL 3rd, Robinson M E, Wade J B, Myers C D.Price DD (2001)Sex differences in negative emotional responses to chronic pain.J. , Pain 2, 354-359.
- 13.Wijnhoven H A.de Vet HC, Picavet HS (2006)Explaining sex differences in chronic musculoskeletal pain in a general population.Pain 124:. 158-166.
- 14.Ahlgren C, Fjellman-Wiklund A, Hamberg K, Johansson E E.Stålnacke BM (2016)The meanings given to gender in studies on multimodal rehabilitation for patients with chronic musculoskeletalpain- a literature review.Disabil. , Rehabil 38, 2255-2270.
- 16.Fillingim R B, Maixner W, Kincaid S, Silva S. (1998) Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. , Pain 75, 121-127.
- 17.Riley JL 3rd, Robinson M E, Wise E A, Myers C D, Fillingim R B. (1998) Sex differences in the perception of noxious experimental stimuli: a meta-analysis. , Pain 74, 181-187.
- 18.Niesters M, Dahan A, Kest B, Zacny J, Stijnen T. (2001) Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. , Pain 151, 61-68.
- 19.Fillingim R B, King C D, Ribeiro-Dasilva M C, Rahim-Williams B, Riley.JL 3rd(2009) Sex, gender, and pain: A review of recent clinical and experimental findings. , J Pain 10, 447-485.
- 20.Bartley E J, Fillingim R B. (2013) Sex differences in pain: A brief review of clinical and experimental findings. , Br J Anaesth 111, 52-58.
- 21.Cbiederman J J, Schefft B K. (1994) Behavioral, physiological, and self-evaluative effects of anxiety on the self-control of pain. , Behav Modif 18, 89-105.
- 23.Popescu A, LeResche L, Truelove E L, Drangsholt M T. (2010) Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. , Pain 150, 309-318.
- 24.Rovner G S, Sunnerhagen K S, Björkdahl A, Gerdle B, Börsbo B.(2017)Chronicpainandsex-differences; women accept and move, while men feel blue.PLoS One 12:e0175737.
- 25.Anand K J. (2000) Pain, plasticity, and premature birth: a prescription for permanent suffering?. , Nat Med 6, 971-973.
- 26.Whitfield M F.Grunau RE (2000)Behavior, pain perception, and the extremely low-birth weight survivor.Clin. , Perinatol 27, 363-379.
- 27.Grunau R E, Holsti L, Haley D W, Oberlander T, Weinberg J. (2005) Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. , Pain 113, 293-300.
- 28.Bhutta A T, Rovnaghi C, Simpson P M, Gossett J M, Scalzo F M. (2001) Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. , Physiol Behav 73, 51-58.
- 30.Walker S M, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. (2003) Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. , Pain 105, 185-195.
- 31.Ren K, Anseloni V, Zou S P, Wade E B, Novikova S I. (2004) Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. , Pain 110, 588-596.
- 32.Wang G, Ji Y, Lidow M S, Traub R J. (2004) Neonatal hind paw injury alters processing of visceral and somatic nociceptive stimuli in the adult rat. , J Pain 5, 440-449.
- 33.Sanada L S, Sato K L, Machado N L, C, Sluka K A.(2014)Cortex glial cells activation, associated with lowered mechanical thresholds and motor dysfunction, persists into adulthood after neonatal pain.Int. , J Dev Neurosci 35, 55-63.
- 34.ALB Simões, GAR Silva, Giorgetto C.de Cassia do Carmo-Campos E, Dias FJ et al (2018)Substance P in dorsal root ganglion neurons in young and adult rats, after nociceptive stimulation during the neonatal period.Anat Rec. 301, 849-861.
- 35.Anand K J, Coskun V, Thrivikraman K V, Nemeroff C B, Plotsky P M. (1999) Long-term behavioral effects of repetitive pain in neonatal rat pups. , Physiol Behav 66, 627-637.
- 36.E C Carmo, Sanada L S, Machado N L.Fazan VPS (2016)Does pain in the neonatal period influence motor and sensory functions in a similar way for males and females during post-natal development in rats?Pain. , Med 17, 1520-1529.
- 37.Pellow S, Chopin P, File S E, Briley M. (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 14, 149-167.
- 38.Brandão M L, Zanoveli J M, Ruiz-Martinez R C, Oliveira L C, Landeira-Fernandez J. (2008) patterns of freezingbehaviororganized in theperiaqueductal grayof rats: association with different types of anxiety.Behav. , Brain Res 188, 1-13.
- 39.Tovote P, Esposito M S, Botta P, Chaudun F, Fadok J P.(2016)Midbrain circuits fordefensivebehaviour.Nature 534:. 206-212.
- 40.Knaepen L, Patijn J, Tibboel D, Joosten.EA (2012)Sex differences in inflammatory mechanical hypersensitivity in later life of rats exposed to repetitive needle pricking as neonates.Neurosci. , Lett 516, 285-289.
- 41.Belviranli M, Atalik K E, Okudan N.Gökbel H (2012)Age and sex affect spatial and emotional behaviors in rats: the role of repeated elevated plus maze test.Neuroscience 227:. 1-9.
- 42.Crombez G, Van Ryckeghem DM, Eccleston C.Van Damme S (2013)Attentional biasto pain-related information: a meta-analysis.Pain 154:. 497-510.
- 43.Sun Z K, Wang J Y.Luo F (2016)Experimental pain induces attentional bias that is modified by enhanced motivation: An eye tracking study.Eur. , J Pain 20, 1266-1277.
- 44.LaPrairie J L.Murphy AZ (2007)Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury.Pain 132 Suppl. 1, 124-133.
- 45.Breslau N, Chilcoat H, Schultz L R. (1998) disorders and the emergence of sex differences in major depression.J. , Gend Specif Med 1, 33-39.
- 46.Tolin D F.Foa EB (2006)Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research.Psychol. , Bull 132, 959-992.
- 47.Bangasser D A.Valentino RJ (2012)Sex differences in molecular and cellular substrates of stress.Cell. , Mol Neurobiol 32, 709-723.
- 48.Leite-Almeida H, Almeida-Torres L, Mesquita A R.Pertovaara A, Sousa N et al (2009)The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats.Pain 144:. 57-65.
- 49.Perrot-Sinal T S, Kostenuik M A, Ossenkopp K P, Kavaliers M. (1996) differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training.Behav. , Neurosci 110, 1309-1320.
- 51.Frankola K A, Flora A L, Torres A K, Grissom E M.Overstreet S et al.(2010)Effects of early rearing conditions on cognitive performance in prepubescent male and female rats.Neurobiol. , Learn Mem 94, 91-99.
- 52.Esmorís-Arranz F J, Méndez C.Spear NE (2008)Contextual fear conditioning differs for infant, adolescent, and adult rats.Behav. , Processes 78, 340-50.
